Periods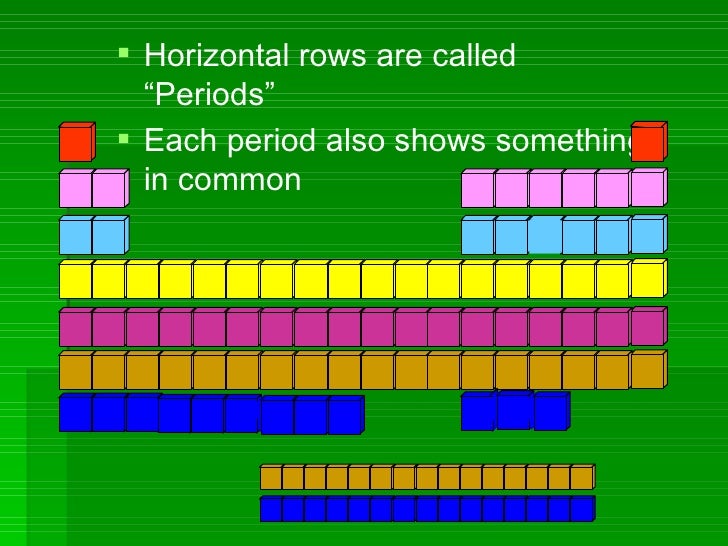

Rows of
elements are called periods.
Groups
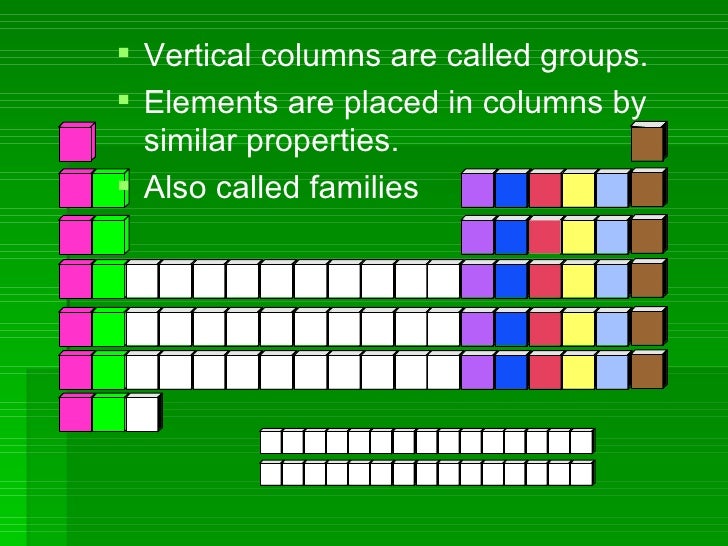


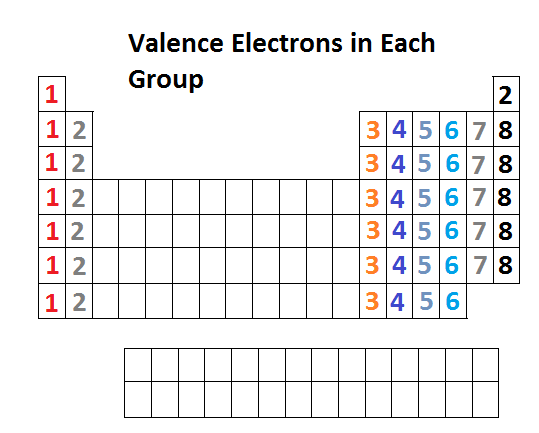
CHARGES ON IONS AND THE PERIODIC TABLE.
The
ion formed by an element can be worked out form the element's position
in the periodic table . The elements of group IV and and group VIII
generally do not form ions.

QUESTIONS
1.In the given periodic table mark
a. one element in group 4
b.a noble gas
c.a transition metal

2.How does the metallic and non-metallic nature of the elements change across period 3 of the periodic table?
REACTIVITIES OF ELEMENTS:
Going from top to bottom of a group in the periodic table ,metals
become more reactive, but non-metals becomes less reactive.












No comments:
Post a Comment