INCOMPLETE COMBUSTION
Incomplete combustion takes place when there is limited supply of oxygen giving poisonous carbon monoxide.
2CH4 + 3O2 ------ 2CO +4 H2O +HEAT ENERGY
Incomplete combustion takes place when there is limited supply of oxygen giving poisonous carbon monoxide.
2CH4 + 3O2 ------ 2CO +4 H2O +HEAT ENERGY

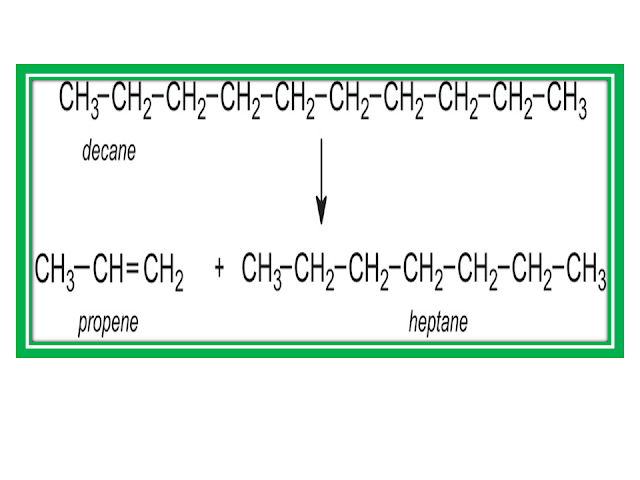
Decane 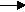 octane + ethene.
octane + ethene.
C10H22(g) 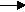 C8H18(g) + C2H4(g)
C8H18(g) + C2H4(g)
Decane 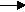 Pentane + ethene.+propene
Pentane + ethene.+propene
C10H22(g)  C5H12(g) + C2H4(g) + C3H6
C5H12(g) + C2H4(g) + C3H6
EXTRA QUESTIONS
1.How does the number of carbon atoms in a hydrocarbon affect its boiling point?.
2.Why can petrol and diesel be separated by fractional distillation?
3.Which of the following is an alkane?a.Ethanol b. Cyclohexane c.Propene
4.Looking at the chemical formulae of
these compounds, which is an alkane?a.C3H8 b. C2H4 c. C5H10
http://chemwiki.ucdavis.edu/Organic_Chemistry/Hydrocarbons/Alkenes
http://www.bbc.co.uk/schools/gcsebitesize/science/ocr_gateway/carbon_chemistry/making_polymersrev2.shtml
EXTRA QUESTIONS
1.Observe the organic compounds given below
Which of these compounds belongs to homologous series
2.Draw any three isomers of hexane.
3. Draw the structure for
b. a branched alkane with five carbon atoms.
4.How do saturated compounds differ from unsaturated compounds in terms of bonding?
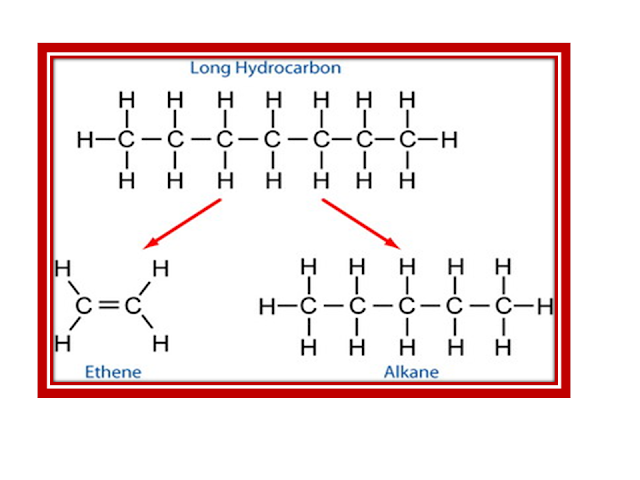
5.Complete the equation for the cracking of C10 H22 to form ethene and one another alkane
http://www.bbc.co.uk/schools/gcsebitesize/science/edexcel/fuels/
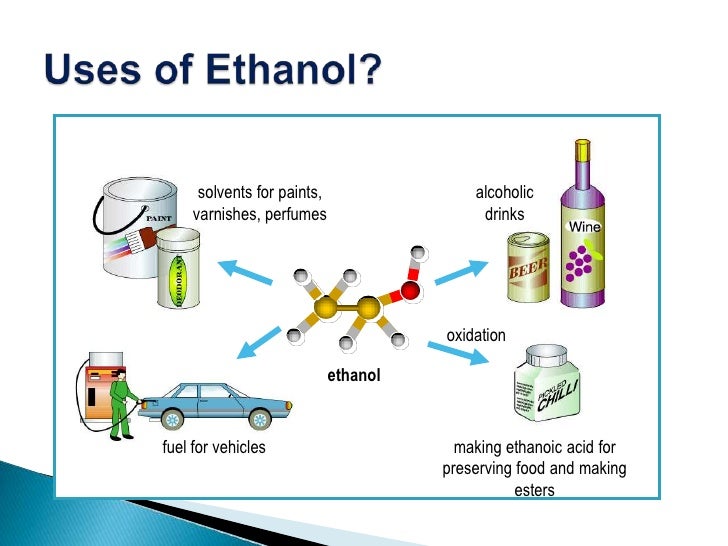
http://www.bbc.co.uk/schools/gcsebitesize/science/triple_ocr_21c/further_chemistry/alcohols/revision/1/
http://www.bbc.co.uk/schools/gcsebitesize/science/triple_ocr_21c/further_chemistry/alcohols/revision/1/






























































































































































No comments:
Post a Comment