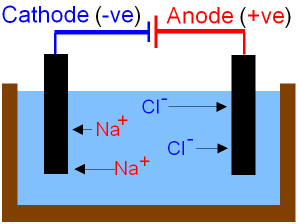
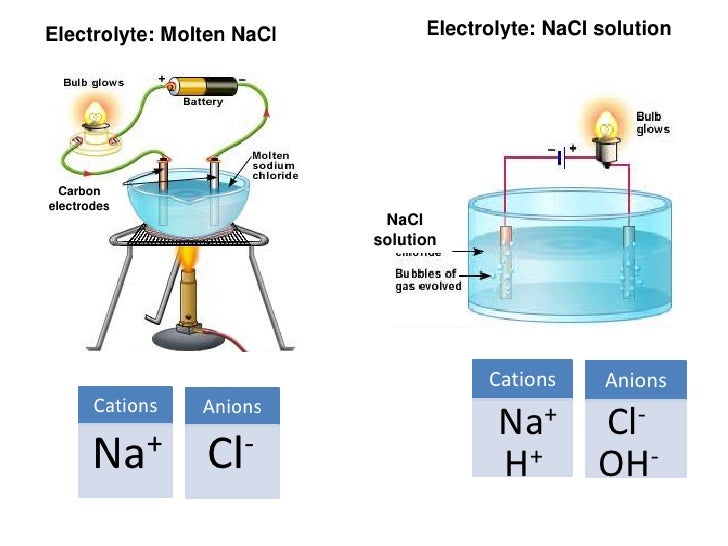
ELECTROLYSIS OF MOLTEN NaCl
ELECTROLYSIS OF SOLUTIONS.

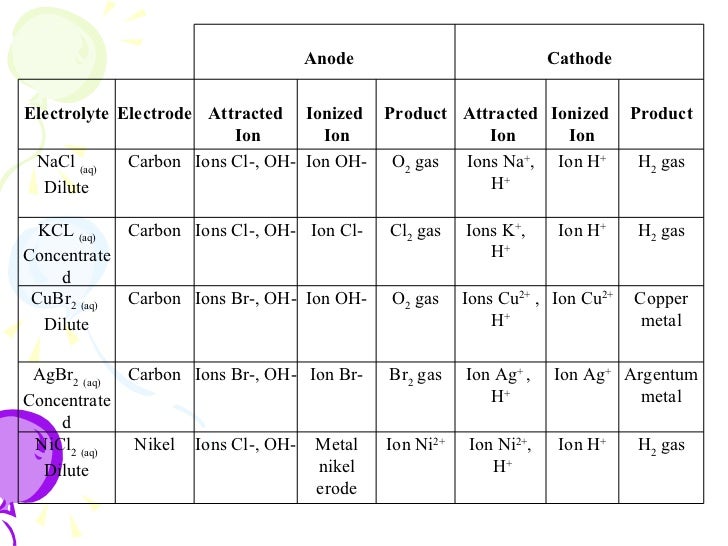

HALF EQUATIONS
At Cathode
- 2H+ + 2e– → H2 (reduction).
- At Anode
4OH- → 2H2O +O2 + 4e– oxidation
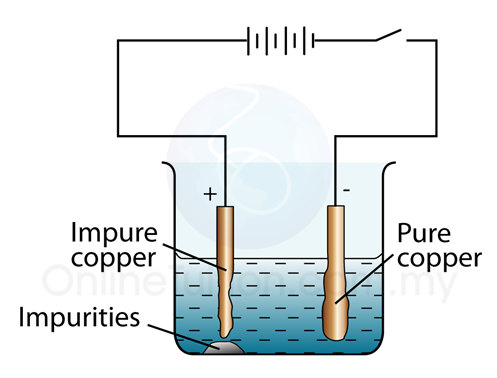


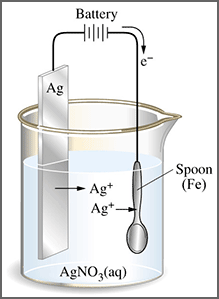

QUESTIONS
1. Mention the anode and cathode in the electroplating of a silver spoon with gold.
2.List any two uses of electroplating.
QUESTIONS
1.List the uses of
a. Aluminium
b. Copper
2. Why aluminium is used in overhead cables?
3.Give reason . Aluminium is used as a building material.
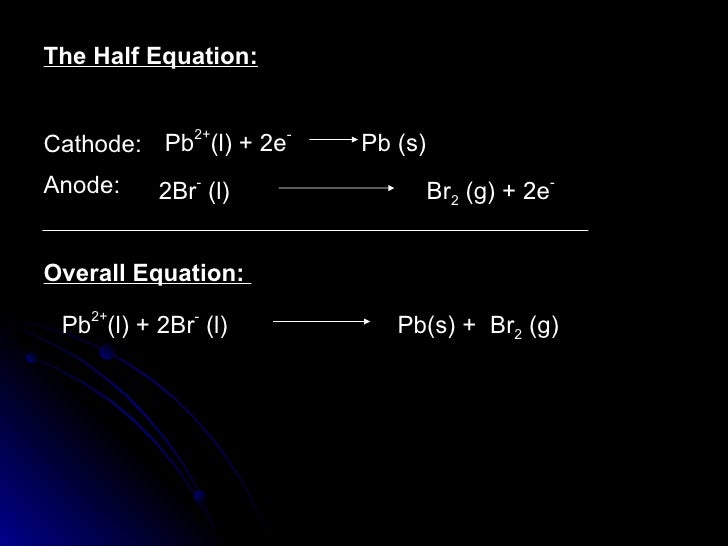
QUESTIONS
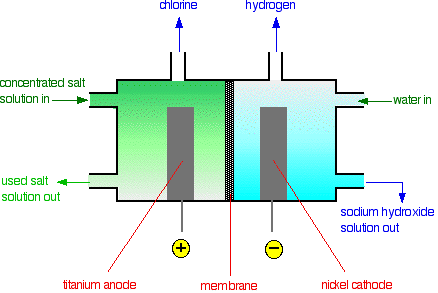
In chlor-alkali process
a. At which electrode chlorine gas is formed?
b. Write a test for chlorine gas.
CHLORINE


















































No comments:
Post a Comment